From Code to Cure: Revolutionizing Traceability and Eradicating Drug Counterfeiting with Technology

- Company
- Domain
- Service
- Technology
- Platform
Problem Statement
Counterfeit drugs are a growing global issue. According to the WHO, counterfeit and substandard drugs constitute over 10% of total sales in low-income and middle-income countries and 1% in developed countries. A review in Forensic Science International (2022) reported that antibiotics, painkillers, and expensive lifestyle pharmaceuticals are frequently targeted by counterfeiters. The WHO estimates that counterfeit drugs cause over one million deaths annually and have a global financial impact of $21 billion. Addressing this challenge, Bilcare approached iauro Systems for a technology solution to ensure drug traceability from source to retail using their patented non-copyable data matrix code.
Solution:
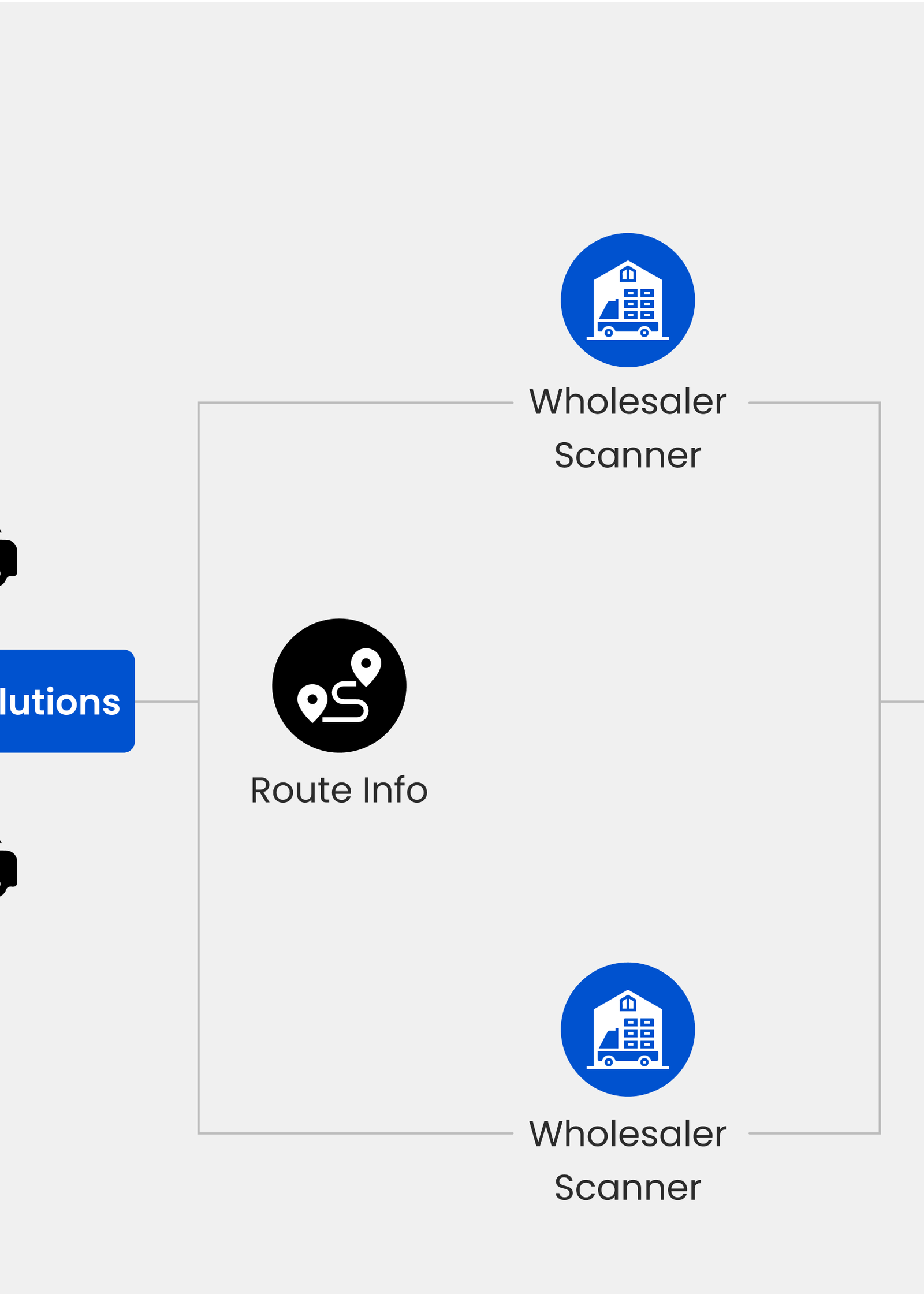
After understanding the problem and the pharmaceutical supply chain, iauro Systems proposed a drug counterfeit avoidance system using a custom data matrix code and package scanning system. This scans the unique data matrix codes on product packaging, at various points—logistics, distributors, and pharmacies—using IoT devices with a scanning application. These scans generate traceability reports, enhancing transparency and reducing the risk of counterfeit drugs entering the market. The solution also includes a dashboard for monitoring product diversion, duplication, and distribution, ensuring comprehensive counterfeit prevention.

Technology Used:
Toolkit
The drug counterfeit avoidance system was built using a robust and scalable technology stack. It employs a Microservices Architecture for flexibility and resilience, and the mobile scanning application is available on both iOS and Android platforms. Continuous integration and deployment are managed with Jenkins. For real-time data collection and processing, Apache Flume, Storm, and Kafka are utilized. The backend services are developed with Java Spring Boot and the MEAN stack (MongoDB, Express.js, Angular, and Node.js). The entire system is hosted on AWS, ensuring security, scalability, and high availability.

Impact :
The drug counterfeit avoidance system has made a significant impact, with over 10 million code scans conducted to date. It is utilized by more than 100 factories, covering 54 different products, and generates and scans over 1,000 unique data-matrix codes per second. The transition of the patented data-matrix code to mobile platforms has enhanced security, making it more difficult for counterfeiters to replicate. As a result, the system has successfully detected over 160 instances of drug counterfeiting, demonstrating its effectiveness in protecting the pharmaceutical supply chain.
From Code to Cure : Revolutionizing Traceability and Eradicating Drug Counterfeiting with Technology

Company
Bilcare
Domain
Logistics
Service
Design Thinking, UI/UX, E2E Product Development, Data Engg
Technology
Angular, JS, CSS3, HTML5, Node.js, Hapi, PostgreSQL, Mongo, Redis, Kafka, PySpark, Redhat
Platform
DSL
Problem Statement
Counterfeit drugs are a growing global issue. According to the WHO, counterfeit and substandard drugs constitute over 10% of total sales in low-income and middle-income countries and 1% in developed countries. A review in Forensic Science International (2022) reported that antibiotics, painkillers, and expensive lifestyle pharmaceuticals are frequently targeted by counterfeiters. The WHO estimates that counterfeit drugs cause over one million deaths annually and have a global financial impact of $21 billion. Addressing this challenge, Bilcare approached iauro Systems for a technology solution to ensure drug traceability from source to retail using their patented non-copyable data matrix code.
Solution:
After understanding the problem and the pharmaceutical supply chain, iauro Systems proposed a drug counterfeit avoidance system using a custom data matrix code and package scanning system. This scans the unique data matrix codes on product packaging, at various points—logistics, distributors, and pharmacies—using IoT devices with a scanning application. These scans generate traceability reports, enhancing transparency and reducing the risk of counterfeit drugs entering the market. The solution also includes a dashboard for monitoring product diversion, duplication, and distribution, ensuring comprehensive counterfeit prevention.


Technology Used:
The drug counterfeit avoidance system was built using a robust and scalable technology stack. It employs a Microservices Architecture for flexibility and resilience, and the mobile scanning application is available on both iOS and Android platforms. Continuous integration and deployment are managed with Jenkins. For real-time data collection and processing, Apache Flume, Storm, and Kafka are utilized. The backend services are developed with Java Spring Boot and the MEAN stack (MongoDB, Express.js, Angular, and Node.js). The entire system is hosted on AWS, ensuring security, scalability, and high availability.

Impact:
The drug counterfeit avoidance system has made a significant impact, with over 10 million code scans conducted to date. It is utilized by more than 100 factories, covering 54 different products, and generates and scans over 1,000 unique data-matrix codes per second. The transition of the patented data-matrix code to mobile platforms has enhanced security, making it more difficult for counterfeiters to replicate. As a result, the system has successfully detected over 160 instances of drug counterfeiting, demonstrating its effectiveness in protecting the pharmaceutical supply chain.
Ready to transform your
business with cutting-edge
software solutions?
Let’s connect and explore
how our expertise can elevate your business