Drug counterfeit avoidance solution
aiding 10 million code scans about the customer

About the customer
Business Need
Implementing a block-chain system enabling transparency in drug record
A dedicated track and trace software system for E2E drugs tracking from origin to the retail store
Supply chain visibility to provide real-time insights to stakeholders
The customer approached us with a patented non-copyable data-matrix code. They recognized the significance of maintaining transparency and accountability in the drug supply chain to ensure patient safety and regulatory compliance. They wanted end-to-end visibility and traceability of drug trials, right from the source to the retail store. With their matrix-code they wanted to capture and record crucial information at every stage. This would empower existing stakeholders, including customers, retailers, and regulatory authorities, to verify the authenticity and track the complete history of drug trials seamlessly.
Enabling traceability offers benefits beyond regulatory compliance. It enhances supply chain visibility, allowing the packaging company and other stakeholders to have real-time insights into the location, status, and movement of drug products. This visibility improves inventory management, reduces the risk of product loss or expiration, and facilitates timely decision-making to streamline operations
Challenges Faced
Integrating the traceability solution with existing system
Ensuring seamless integration between their packaging machinery, inventory management systems, supply chain software
Ensuring standardization and interoperability
Between different systems, data formats, and communication protocols
Ensuring scalability and volume
Strengthening the solution to handle increasing volume of packages, data, and transactions while maintaining performance and response time.
Standardization and visibility into the entire system
Achieving traceability by collaborating with various stakeholders, including drug manufacturers, distributors, logistics providers, and retail stores
A unique solutioning
approach to start with.
- A blockchain-based system that can provide an immutable and transparent record of drug trial traceability
- A dedicated track and trace software system can streamline the process of tracing drug trials
- A platform that utilizes the patented non-copyable data-matrix code can empower customers, retailers, and other stakeholders to verify the authenticity and trace the drug trial history
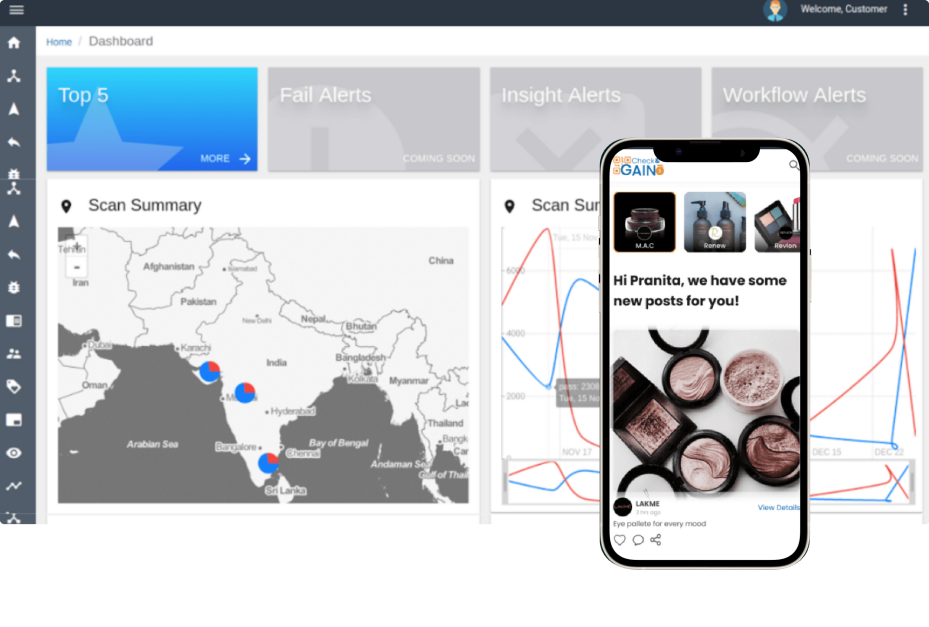
The client wanted us to integrate their patented data matrix code into the technological solution that we curated for them, and to fuse it together we incorporated the following features into the platform that we built
Drug Counterfeit Avoidance System
The initial phases of preventing the production and distribution of counterfeit drugs are done by building a Drug Counterfeit System that detect counterfeit attempts and ensure only genuine products reach the market. This system included features like tamper-evident packaging, RFID (Radio-Frequency Identification) tags and other counterfeiting measures. This aids in maintaining the authenticity of the products that the client handles.
Custom Data-Matrix Code & Package Scanning System
This involves the use of unique identifiers, such as bar codes, QR codes, on individual drug packages, which can be linked to the drugs database and then by scanning these codes at different stages of the supply chain, stakeholders can verify the authenticity and trace the entire journey of the drug from source to retail store. This system provides transparency and ensures that counterfeit or diverted products can be quickly identified and removed from circulation.
The customer approached us with a patented non-copyable data-matrix code. They recognized the significance of maintaining transparency and accountability in the drug supply chain to ensure patient safety and regulatory compliance. They wanted end-to-end visibility and traceability of drug trials, right from the source to the retail store. With their matrix-code they wanted to capture and record crucial information at every stage. This would empower existing stakeholders, including customers, retailers, and regulatory authorities, to verify the authenticity and track the complete history of drug trials seamlessly.
Enabling traceability offers benefits beyond regulatory compliance. It enhances supply chain visibility, allowing the packaging company and other stakeholders to have real-time insights into the location, status, and movement of drug products. This visibility improves inventory management, reduces the risk of product loss or expiration, and facilitates timely decision-making to streamline operations
Advanced product diversion,
duplication and distribution dashboards
All of this culminated into an advanced dashboard that provides real-time insights and analytics, related to product diversion, duplication, and distribution patterns. These dashboards utilize data collected from various sources, such as the scanning system, inventory management systems, and external data feeds, to identify any irregularities or anomalies in the supply chain. This data can be like unauthorized distribution, parallel trade, or duplicate packaging monitored by our clients to proactively detect and address issues like unauthorized distribution, parallel trade, or duplicate packaging.
End Impact
The platform that we built as result of the business need of the user proved not just to be scalable but also efficient in handling data traceability, including scanning and tracking of drug products across a vast network of factories and products. The transition to mobile accessibility and enhanced security of the patented data-matrix code further enhances the system’s effectiveness. The boosted security also implies the sound measures that have been undertaken to safeguard the integrity and authenticity of the code, making it more resistant to tampering or counterfeiting attempts.
Thus, the high number of detections underscores the importance of robust traceability measures in combating drug counterfeiting.
Enabled 10+ million code scans
100+ factories, 54+ products, 1000+ unique data-matrix codes generated and scanned per second
Patented data-matrix code transitioned to mobility and hence enhanced security
160+ drug counterfeit instances detected

Implementing a block-chain system enabling transparency in drug record
A dedicated track and trace software system for E2E drugs tracking from origin to the retail store
Supply chain visibility to provide real-time insights to stakeholders
The customer approached us with a patented non-copyable data-matrix code. They recognized the significance of maintaining transparency and accountability in the drug supply chain to ensure patient safety and regulatory compliance. They wanted end-to-end visibility and traceability of drug trials, right from the source to the retail store. With their matrix-code they wanted to capture and record crucial information at every stage. This would empower existing stakeholders, including customers, retailers, and regulatory authorities, to verify the authenticity and track the complete history of drug trials seamlessly.
Enabling traceability offers benefits beyond regulatory compliance. It enhances supply chain visibility, allowing the packaging company and other stakeholders to have real-time insights into the location, status, and movement of drug products. This visibility improves inventory management, reduces the risk of product loss or expiration, and facilitates timely decision-making to streamline operations
Integrating the traceability solution with existing system
Ensuring seamless integration between their packaging machinery, inventory management systems, supply chain software
Ensuring standardization and interoperability
Between different systems, data formats, and communication protocols
Ensuring scalability and volume
Strengthening the solution to handle increasing volume of packages, data, and transactions while maintaining performance and response time.
Standardization and visibility into the entire system
Achieving traceability by collaborating with various stakeholders, including drug manufacturers, distributors, logistics providers, and retail stores
- A blockchain-based system that can provide an immutable and transparent record of drug trial traceability
- A dedicated track and trace software system can streamline the process of tracing drug trials
- A platform that utilizes the patented non-copyable data-matrix code can empower customers, retailers, and other stakeholders to verify the authenticity and trace the drug trial history
Drug Counterfeit Avoidance System
The initial phases of preventing the production and distribution of counterfeit drugs are done by building a Drug Counterfeit System that detect counterfeit attempts and ensure only genuine products reach the market. This system included features like tamper-evident packaging, RFID (Radio-Frequency Identification) tags and other counterfeiting measures. This aids in maintaining the authenticity of the products that the client handles.
Custom Data-Matrix Code & Package Scanning System
This involves the use of unique identifiers, such as bar codes, QR codes, on individual drug packages, which can be linked to the drugs database and then by scanning these codes at different stages of the supply chain, stakeholders can verify the authenticity and trace the entire journey of the drug from source to retail store. This system provides transparency and ensures that counterfeit or diverted products can be quickly identified and removed from circulation.
The customer approached us with a patented non-copyable data-matrix code. They recognized the significance of maintaining transparency and accountability in the drug supply chain to ensure patient safety and regulatory compliance. They wanted end-to-end visibility and traceability of drug trials, right from the source to the retail store. With their matrix-code they wanted to capture and record crucial information at every stage. This would empower existing stakeholders, including customers, retailers, and regulatory authorities, to verify the authenticity and track the complete history of drug trials seamlessly.
Enabling traceability offers benefits beyond regulatory compliance. It enhances supply chain visibility, allowing the packaging company and other stakeholders to have real-time insights into the location, status, and movement of drug products. This visibility improves inventory management, reduces the risk of product loss or expiration, and facilitates timely decision-making to streamline operations